When you’re pregnant, even a simple headache can turn into a crisis. You reach for that painkiller, but then you stop - is it safe? You’re not alone. Nearly 9 out of 10 pregnant women take at least one medication during pregnancy, and half take four or more. Yet most of these drugs were never tested on pregnant people. That’s not an oversight - it’s the rule. Clinical trials almost always exclude pregnant women, leaving doctors and patients guessing. That’s where safety alerts come in - not as warnings to avoid all meds, but as tools to help you make smarter, safer choices.
Why the Old Letter System Failed
You might remember the old FDA pregnancy categories: A, B, C, D, X. They looked simple. A was safe. X was dangerous. But they didn’t tell the whole story. A Category C drug didn’t mean it was risky - it just meant there wasn’t enough data. Many women stopped taking essential medications like antidepressants or thyroid pills because they saw a “C” and assumed danger. A 2019 study at Massachusetts General Hospital found that after the letter system was dropped in 2015, unnecessary medication discontinuation dropped by 18%. That’s huge. The old labels didn’t protect women - they scared them into harm.What Replaced the Letters
Today, drug labels don’t use letters. They use stories. The FDA’s Pregnancy and Lactation Labeling Rule (PLLR), fully in effect since 2018, requires drug makers to explain three things clearly: risks during pregnancy, risks while breastfeeding, and how the drug affects fertility. This isn’t just fine print - it’s detailed narrative. For example, the label for valproate (used for epilepsy and bipolar disorder) now clearly states: “Use during pregnancy can increase the risk of neural tube defects from 0.1% to 1-2%.” That’s specific. That’s useful. But here’s the catch: only 32% of these labels include actual numbers. Most still say things like “may cause harm,” without telling you how often or how badly.How Safety Alerts Are Triggered
Safety alerts don’t appear out of nowhere. They’re born from data - or the lack of it. The FDA tracks 38 active pregnancy exposure registries, where doctors and patients report what medicines were taken and what happened to the baby. But here’s the problem: less than 1% of all pregnancies involving medication are captured in these registries. That means for most drugs, we’re flying blind for years. A 2020 study found it takes an average of 7.2 years to confirm a drug’s risk after it’s already widely used in pregnancy. That’s why alerts often come too late. The EMA in Europe takes a more proactive approach. For high-risk drugs like lenalidomide, they require mandatory pregnancy tests and birth control for six months after stopping the drug. The U.S. doesn’t have that level of enforcement - yet.
What Drugs Are Actually Dangerous
Some medications are known to cause serious birth defects. Isotretinoin (Accutane), used for severe acne, causes defects in 20-35% of exposed pregnancies. Valproate increases the risk of spina bifida and cognitive delays. Thalidomide - infamous for the 1960s birth defect crisis - is still used today for certain cancers, under strict controls. But the bigger danger isn’t just the obvious ones. It’s the ones we assume are safe. Common OTC painkillers like ibuprofen can reduce amniotic fluid and affect fetal heart development if taken after 20 weeks. Even some herbal supplements - like black cohosh or dong quai - can trigger contractions. The real issue? Most women don’t know what’s in their medicine cabinet. Only 37% of pregnant women check the pregnancy safety section on drug labels, according to CDC data.What’s Safe - and What to Keep Taking
The fear of medication often causes more harm than the meds themselves. Untreated depression, high blood pressure, asthma, or thyroid disease can be far more dangerous to both mother and baby than the drugs used to manage them. The American College of Medical Toxicology says it plainly: “No medication is 100% safe - but untreated illness can be worse.” A 2022 report from Mass General’s Pregnancy Medication Safety Hotline found that 78% of calls were about anxiety or depression meds - and in 63% of those cases, the advice was to keep taking the medication. Folic acid? Absolutely continue. 800 mcg daily through at least 12 weeks cuts neural tube defect risk by up to 70%. Acetaminophen? Still the top choice for pain and fever. Insulin? Essential for gestational diabetes. These aren’t risky - they’re lifesaving. The key is knowing which ones belong in your pregnancy toolkit.What You Can Do Right Now
Don’t wait for your next appointment. Start now. Make a list of everything you take - prescriptions, OTC pills, vitamins, herbal teas, even CBD gummies. Bring it to your first prenatal visit. ACOG recommends a full medication reconciliation - and it takes about 22 minutes. Ask your provider: “Is this still needed? Is there a safer alternative? What happens if I stop?” If you’re on a high-risk drug like lithium or methotrexate, ask if there’s a registry you can join. Use the FDA’s Medicine and Pregnancy webpage - it’s one of the few trusted, plain-language sources with 4.3 out of 5 stars from users. Avoid relying on Reddit threads or drug review sites. One in two pregnant women report conflicting advice online. That’s not helpful - it’s terrifying.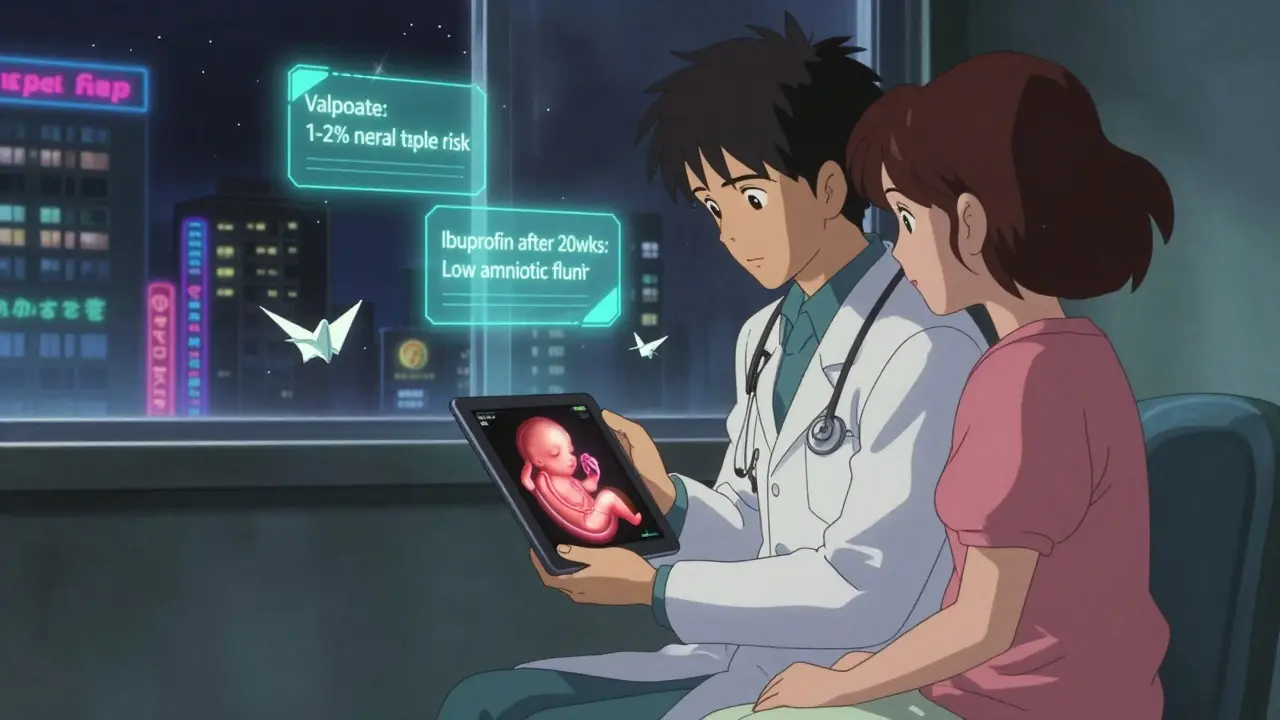
The Bigger Picture: Why This System Is Broken
We spend $1.27 billion a year on pregnancy medication safety tools - and it’s growing. But the system is still broken. Only 22% of drug companies maintain the pregnancy registries they’re required to run. Just 28% of U.S. hospitals have electronic systems that automatically flag risky meds when a woman is pregnant. And the NIH’s new $25 million PREGNET initiative, which aims to track 100,000 pregnancies, won’t even start seeing results until 2029. Meanwhile, the March of Dimes estimates a $312 million annual funding gap through 2030. Without more investment, we’ll keep reacting to tragedies instead of preventing them. The technology exists - AI tools can predict risks with 70% accuracy using real-world data - but we’re not using it at scale.What’s Coming Next
The FDA is now piloting weekly safety reporting for high-risk drugs like mycophenolate mofetil - instead of waiting three months. The EMA is expanding mandatory contraception rules for 12 more drugs. And digital tools are finally catching up: 63% of big pharma companies are building pregnancy-specific apps. But only 12% have real user engagement. The future isn’t just better labels - it’s better data. Real-time tracking. AI-driven alerts. Integrated health records that know you’re pregnant and warn you before you pick up that prescription. But until then, your best tool is knowledge - and asking questions.Can I take ibuprofen while pregnant?
Avoid ibuprofen after 20 weeks of pregnancy. It can reduce amniotic fluid and affect your baby’s heart development. Before 20 weeks, occasional use is usually okay, but acetaminophen is still the safer first choice. Always check with your provider before taking any NSAID.
Are antidepressants safe during pregnancy?
For many women, yes - and stopping them can be riskier than continuing. SSRIs like sertraline and citalopram have the most data supporting their use. Untreated depression increases risks of preterm birth, low birth weight, and postpartum complications. If you’re stable on your current medication, your provider will likely recommend staying on it. Never stop cold turkey - withdrawal can cause serious symptoms.
What if I took a risky medication before I knew I was pregnant?
Don’t panic. Most medications don’t cause harm in the first few weeks - before the embryo implants. If you took something like isotretinoin or thalidomide, contact your OB or a teratology specialist immediately. For other drugs, the risk is often very low. Your provider can help assess timing, dosage, and potential effects. Many women have taken risky meds unknowingly and gone on to have healthy babies.
Is it safe to take vitamins and supplements during pregnancy?
Prenatal vitamins with folic acid (800 mcg), iron, and DHA are recommended. But not all supplements are safe. Avoid high-dose vitamin A, herbal teas like chamomile or peppermint in large amounts, and unregulated products like “pregnancy teas” or detox supplements. Always check labels and talk to your provider - even “natural” doesn’t mean safe.
Where can I find trustworthy information about pregnancy medications?
Stick to trusted sources: the FDA’s Medicine and Pregnancy page, MotherToBaby (a service of the Organization of Teratology Information Specialists), and your OB’s office. Avoid drug review sites, social media groups, or blogs. These often mix anecdotal stories with facts, leading to confusion. MotherToBaby also offers free, confidential phone consultations - they answered over 12,000 calls in 2022.