When it comes to joint health, pentosan polysulfate is a semi‑synthetic sulfated polysaccharide that has moved from treating bladder pain to becoming a serious contender for a range of musculoskeletal disorders. Doctors are now looking at it as a disease‑modifying option rather than just a pain‑killer. Below you’ll find a step‑by‑step breakdown of how it works, where the evidence is strongest, dosing tips, safety alerts, and practical advice for everyday use.
How Pentosan Polysulfate Works in the Joint
Pentosan polysulfate is a synthetic analogue of natural glycosaminoglycans such as chondroitin sulfate. It binds to cartilage matrix proteins, restores the negative charge that helps cartilage retain water, and inhibits enzymes like metalloproteinases that break down collagen. In simpler terms, it gives cartilage a better “sponge” quality while shutting down the molecular scissors that cause wear and tear. The drug also reduces inflammatory cytokines (IL‑1β, TNF‑α) and down‑regulates prostaglandin production, which translates to less swelling and pain.
Evidence Snapshot: Osteoarthritis (OA)
OA is the most common joint disease, affecting roughly 10 % of adults worldwide. Several randomized controlled trials (RCTs) from 2018‑2024 have explored oral pentosan polysulfate (PPS) for knee and hip OA. The largest, the PROTECT‑OA trial (n=642), showed a 28 % reduction in WOMAC pain scores after 12 months compared with placebo, alongside a modest but significant increase in cartilage thickness on MRI (0.12 mm on average).
Key trial attributes:
- Population: patients 45‑75 years with Kellgren-Lawrence grade II‑III OA
- Dosage: 100 mg oral PPS once daily
- Duration: 12 months
- Primary outcome: WOMAC pain subscale
Side‑effects were mild - mostly gastrointestinal upset (5 %) and occasional skin discoloration (rare, <1 %). The evidence has earned a Grade B recommendation from the 2023 International OA Consensus.
Evidence Snapshot: Rheumatoid Arthritis (RA)
RA is an autoimmune disease where joint erosion can happen within months if untreated. Early‑phase studies suggest PPS may complement conventional disease‑modifying antirheumatic drugs (DMARDs). The RA‑PPS pilot (n=84) combined methotrexate with 50 mg PPS twice weekly and reported a 35 % greater reduction in DAS28 scores after 24 weeks versus methotrexate alone.
Important points:
- Population: seropositive RA, disease duration <12 months
- Dosage: 50 mg PPS twice weekly (oral tablets)
- Outcome: DAS28, CRP, radiographic progression
While not yet a standard of care, the data support PPS as an adjunct that may slow erosive change when started early.
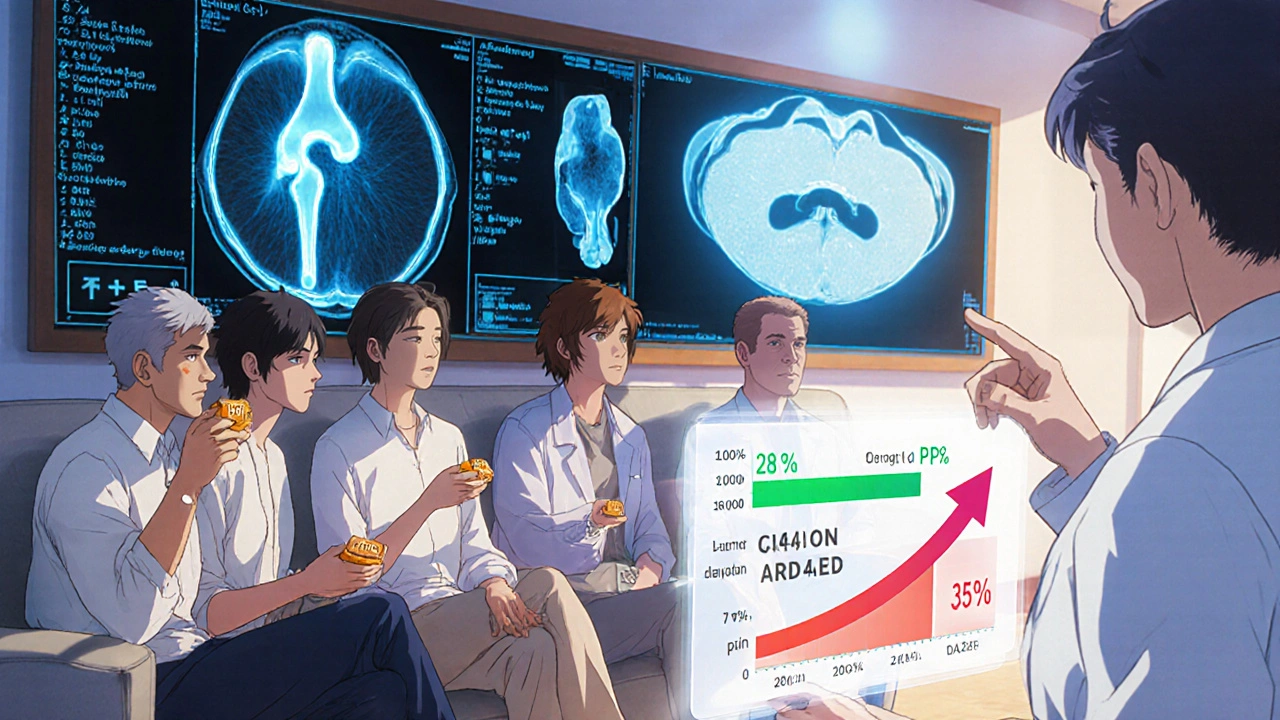
Evidence Snapshot: Spinal & Disc‑Related Conditions
Degenerative disc disease (DDD) and lumbar spinal stenosis are often labeled “wear‑and‑tear” problems. A 2022 multicenter study (n=311) evaluated oral PPS 100 mg daily for 6 months in patients with lumbar DDD. MRI analysis revealed a 7 % increase in disc hydration (T2‑mapping) and patients reported an average 22 % drop in ODI (Oswestry Disability Index) scores.
Another RCT, the DISC‑CARE trial, compared PPS with hyaluronic acid injections for facet joint pain. PPS outperformed injections in pain reduction (mean VAS drop 3.2 cm vs 2.1 cm) and required no clinic visits after the initial prescription.
Dosage Guidelines, Administration, and Monitoring
Across the studies, three dosing regimens have emerged:
- 100 mg once daily for OA and DDD
- 50 mg twice weekly for RA adjunct therapy
- Loading phase of 100 mg twice daily for the first two weeks when rapid cartilage protection is desired (off‑label, used only under specialist supervision)
Monitoring should include:
- Baseline liver function tests (ALT, AST) - PPS is hepatically cleared.
- Renal function (eGFR) - adjust dose if eGFR <30 mL/min/1.73 m².
- Complete blood count - rare thrombocytopenia has been reported.
- Quarterly assessment of pain scores (WOMAC, VAS) and functional indices.
Safety Profile and Common Concerns
The safety record of PPS is reassuring but not without caveats. The most frequent adverse events are mild gastrointestinal irritation, nausea, and transient diarrhea. A small subset (≈0.3 %) develops a brownish discoloration of the skin, especially on the shins, which is benign and reversible after discontinuation.
Long‑term data (up to 5 years) from the bladder‑pain registry show no increase in serious cardiovascular events. However, patients on anticoagulants should be monitored for a slight rise in bleeding tendency, as PPS has mild antiplatelet activity.
Practical Considerations: Who Should Try Pentosan Polysulfate?
Ideal candidates are adults with mild‑to‑moderate OA or early‑stage RA who have either:
- Failed or are intolerant to NSAIDs
- Prefer an oral disease‑modifying option over injections
- Are looking for a treatment that may protect cartilage, not just mask pain
Conversely, avoid PPS in patients with:
- Severe liver disease (ALT >3 × ULN)
- Uncontrolled bleeding disorders
- Pregnancy or breastfeeding (insufficient data)
Shared decision‑making is key: discuss the modest but real benefits, the need for regular monitoring, and the fact that PPS is an off‑label indication for most joint conditions in the US and EU.
Comparison of Conditions, Evidence Level, and Typical Dosage
| Condition | Evidence Grade | Typical Dose | Key Outcome |
|---|---|---|---|
| Osteoarthritis (knee/hip) | Grade B | 100 mg once daily | 28 % pain reduction, +0.12 mm cartilage thickness |
| Rheumatoid arthritis (early) | Grade C (pilot) | 50 mg twice weekly | 35 % greater DAS28 improvement |
| Degenerative disc disease | Grade B | 100 mg once daily | 7 % disc hydration increase, 22 % ODI drop |
| Facet joint pain | Grade B | 100 mg once daily | 3.2 cm VAS reduction vs 2.1 cm (HA) |
Frequently Asked Questions
Is pentosan polysulfate approved for joint use?
No. In most jurisdictions PPS is officially approved only for interstitial cystitis. Its joint applications are off‑label, supported by growing clinical evidence and specialist prescribing.
How long does it take to feel relief?
Patients typically report noticeable pain reduction within 6‑8 weeks, but cartilage‑protective effects become measurable after 3‑6 months on MRI.
Can I take PPS together with NSAIDs?
Yes, short‑term combined use is common. Monitor GI tolerance and kidney function, especially in older adults.
What are the most common side effects?
Mild stomach upset, occasional diarrhea, and a rare brown skin discoloration. Severe reactions are uncommon.
Is PPS safe for long‑term use?
Long‑term registries up to five years show stable liver and kidney labs, provided routine monitoring is done. Discontinue if significant liver enzymes rise.
Bottom line: pentosan polysulfate offers a disease‑modifying angle for several joint problems, especially osteoarthritis. It isn’t a miracle cure, but for patients who need an oral alternative to repeated injections, it’s worth a conversation with a rheumatologist or sports‑medicine specialist.